Our Science
The TME is a complex ecosystem of cells and non-cellular components that both surrounds and penetrates within solid tumors. The TME influences tumor development, tumor immunity, metastasis, and response to anti-tumor therapies.
In solid cancers, the TME presents structural and biochemical barriers that can inhibit immune cell access to tumor cells, block immune cell activation, and diminish the effectiveness of treatment, including with immunotherapies. Response rates, including progression-free survival, remain very low across many treatments for solid cancers, particularly among advanced patients, in cancers such as thymic, pancreatic, ovarian, colorectal, lung and breast.
Incendia believes that there are multiple novel opportunities within the TME to target treatment of cancer and improve outcomes.
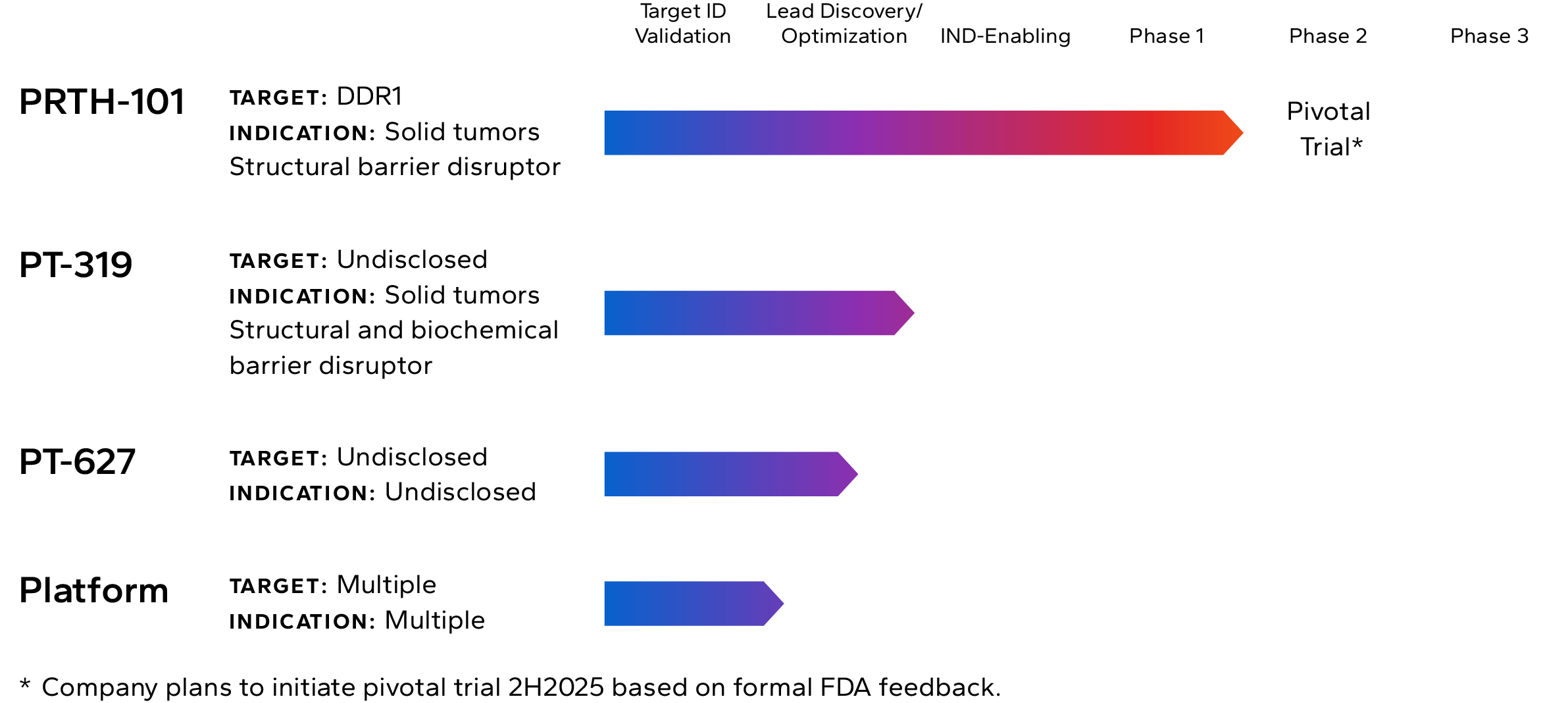
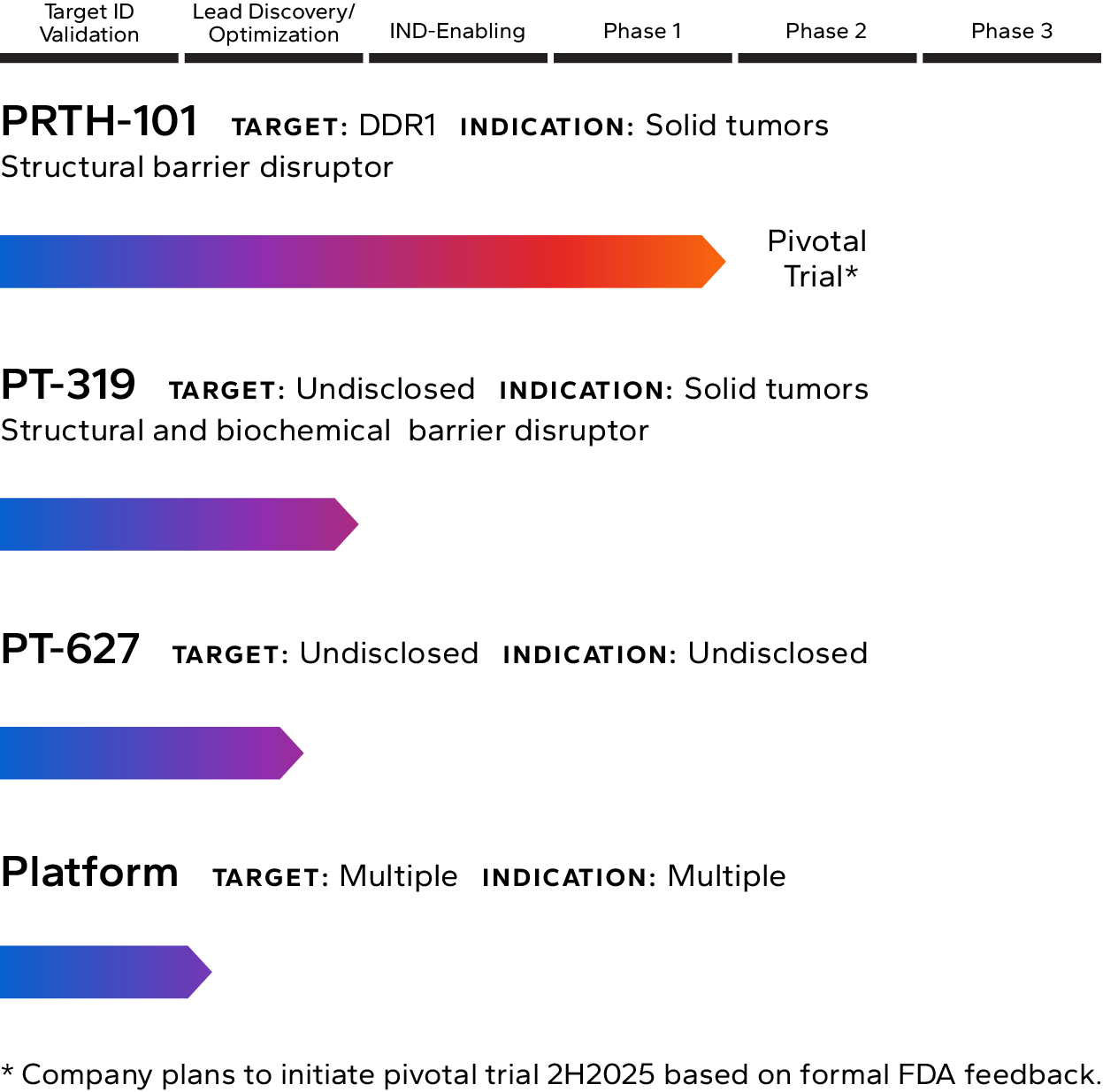
Lead Pipeline Asset
Incendia’s most advanced investigational therapeutic is PRTH-101, a novel therapeutic antibody that binds to and inhibits Discoidin Domain Receptor 1 (DDR1), an element of the TME that is highly expressed by cancer cells in multiple solid tumor types.
The Company is currently recruiting patients into a Phase 1c clinical trial with PRTH-101 alone and in combination with PD-1 inhibition for the treatment of patients with ICI refractory thymic carcinoma (NCT05753722). The company intends to start a pivotal Phase 2 trial of PRTH-101 in the second half of 2025.